Classification of Matter

* Chemistry is the branch of science that deals with the study of the composition, structure, properties of matter and changes it undergoes.
* Anything that has mass and occupies space is known as matter.
* Matter can exist in three physical states based on the arrangement of constituent particles – solid, liquid and gaseous.
* Matter can be classified as pure substances and mixtures based on the chemical composition.
* Based on the uniformity of the particles, mixture can further be classified as homogenous and heterogeneous mixture.
* Pure substances can be classified into elements and compounds based on the possibility to separate the substance into its constituents.
Properties of Matter
* Physical properties are those properties which can be measured or observed without changing the identity or the composition of the substance.
* The chemical properties of a substance can be observed when the substance undergoes a chemical reaction.
* Mass is the amount of matter present in a substance, while weight is the gravitational force exerted on the substance.
* Mass of a substance remains constant, whereas its weight varies from place to place.
* Density is the amount of mass per unit volume of the substance.
* Temperature in Kelvin (K) = Temperature in Celsius (C) + 273°C
= \frac{5}{9}(°F-32) or °F = \frac{9}{5} (°C) + 32
Uncertainty in Measurement

* In scientific notation, any number can be represented as N multiplied by ten to the power of n, where lowercase n is an exponent having positive or negative values, and uppercase N is a coefficient that can vary between one and ten.
* If the average value of measurements is close to the correct value, the measurement is said to be accurate.
* If the values of measurement are close to each other and hence close to their average value, the measurement is said to be precise.
* If is important to indicate the level of certainty and uncertainty in any measurement as it will affect the output or result of the experiment.
* The certainty and uncertainty in a measurement is indicated through the use of significant figure.
* Significant figure is the total number of digits in a number, including the last digit whose value is uncertain.
* Dimensional analysis is used to convert a given quantity from one type of unit to another unit.
Laws of Chemical Combination and Dalton’s Atomic Theory
* There are the five basic laws of chemical combination that govern every chemical reaction. They are:
• Laws of Conservation of Mass.
• Law of Constant Composition or Definite proportions.
• Law of Multiple Proportions.
• Law of Combining volumes (Gay-Lussac’s Law of Gaseous Volumes).
• Avogadro’s Law.
* Law of Conservation of mass states that in all physical and chemical changes, the total mass of the reactants is equal to the total mass of the products.
* Law of definite proportions states that a pure chemical compound always contains the same elements combined together in the same fixed ratio by mass.
* Law of multiple proportions states that when two elements combine to form more than one chemical compound, then the masses of one of the elements that combine with a fixed mass of the other element, bears a simple ratio.
* Gay Lussac’s law of combining volumes states that when gases combine or are produced, under similar conditions of temperature and pressure, volumes of reacting gases as well as the products bear a simple ratio to one another.
* Avogadro’s Law states that under similar conditions of temperature and pressure, equal volume of all gases contains equal number of molecules.
 * Dalton’s Atomic Theory put forward the following points:
* Dalton’s Atomic Theory put forward the following points:
• Matter consists of extremely small indivisible and indestructible particles called atoms.
• Atoms of the same elements are identical in shape, size and mass.
• Atoms of different elements have different sizes, masses and chemical properties.
• Atoms of two or more elements combine in a fixed ratio to form a compound.
• Atoms can neither be created nor be destroyed during chemical reaction.
• Chemical reactions involve combination, separation or rearrangement of atoms.
* The Modern Atomic Theory put forward the following points:
• An atom is no longer considered indivisible- it is made up electrons, protons and neutrons.
• Atoms of the same element may have different atomic masses.
• Atoms of elements that possess different atomic masses are known as isotopes.
• Atoms of different elements may have same atomic masses.
• The ratio in which different atoms of different elements combine may be fixed and integral, but may not always be simple.
• The atom is the smallest particle that takes part in a chemical reaction.
• The atom is not indestructible.
Atomic and Molecular Masses
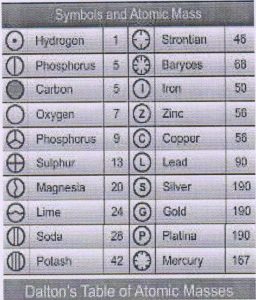 The atomic mass of an element is the number of times an atom of that element is heavier than an atom of carbon takes as 12.
The atomic mass of an element is the number of times an atom of that element is heavier than an atom of carbon takes as 12.
* One atomic mass unit is defined as a mass equal to \frac{1}{12}th the mass of carbon – 12 atom.
* Average atomic mass of an isotope is the sum of abundance of each isotope multiplied by precise atomic mass.
* Fractional abundance of an isotope is the fraction of total number of atoms that is comprised of that particular isotope.
* Average atomic mass can also be calculated as the sum of the products of fractional abundance of the isotopes and their corresponding mass numbers.
* When the atomic mass of an element is expressed in grams, it is called gram atomic mass. Gram atomic mass is also called one gram atom of the element.
* The molecular mass of substance (elements or compound) is the number of times the molecule of the substance is heavier than \frac{1}{12}th the mass of an atom of carbon-12.
* The molecular mass of any elements or compound cab be calculated by adding the atomic mass of all the atoms present in one molecule of that element or compound.
* When the molecular mass is expressed in grams, it is known as gram molecular mass.
* Gram molecular mass is also called one gram molecule of the substance.
* Formula mass is the sum of atomic masses of atoms present in one formula unit of an ionic crystal.
* When the formula mass is expressed in grams, it is known as gram formula mass.
Mole Concept and Percentage Composition
* Mole is the amount of substance, which contains as many elementary entities as there are in 12 g of carbon.
* One mole = 6.022 x 10^{23}chemical units.
* Avogadro’s number or Avogadro’s constant is the number of elementary entities (atoms or molecules or ions) in one mole of a substance.
* Molar mass is the mass of one mole of substance, expressed in grams. The molar mass is numerically equivalent to the atomic (or molecular or formula) mass in u.
* The percentage of any element or constituent in a compound is the number of parts by mass of that element or constituent present in 100 parts by mass of the compound.
* The empirical formula gives the simplest whole number ratio of the different atoms present in one molecule of the compound.
* The molecular formula gives the actual number of atoms of various elements present in one molecule of the compound.
Stoichiometry and Stoichiometric Calculations
* A chemical equation in which the number of atoms of each element is equal on the reactants side and the products side is called a balanced chemical equation.
* Steps to balance a chemical equation:
• Write the chemical formula for the reactants and products.
• Balance the number of atoms of each one by one.
• Verify that the number of atoms in each element is balanced.
* A balanced equation helps determine the quantitative relationship between the reactants and products in terms of number of moles, molecules, atomic masses and volumes.
* The calculation of quantitative relationships of the reactants and products in a balanced chemical reaction is known as stoichiometry.
* The coefficients in a balanced chemical equation are called stoichiometric coefficients.
* The mass percent of a solution is determined by dividing the mass of the solute by the mass of the solution and multiplying it by hundred.
* Mole fraction is the ratio of number of moles of a particular component to the total number of moles of the solution.
* Molarity (M) is defined as the number of moles of solute in one litre of solution.
* Molality (m) is defined as the number of moles of a solute present in 1 kg of solvent.
